Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 C based on two binary non-ideal solid solutions
C based on two binary non-ideal solid solutionsWalker, C.; Suto, Shunkichi; Oda, Chie; Mihara, Morihiro; Honda, Akira
Cement and Concrete Research, 79, p.1 - 30, 2016/01
Times Cited Count:69 Percentile:90.42(Construction & Building Technology)Modeling the solubility behavior of calcium silicate hydrate (C-S-H) gel is important to make quantitative predictions of the degradation of hydrated ordinary Portland cement (OPC) based materials. Experimental C-S-H gel solubility data have been compiled from the literature, critically evaluated and supplemented with new data from the current study for molar Ca/Si ratios = 0.2-0.83. All these data have been used to derive a discrete solid phase (DSP) type C-S-H gel solubility model based on two binary non-ideal solid solutions in aqueous solution(SSAS). Features of the DSP type C-S-H gel solubility model include satisfactory predictions of pH values and Ca and Si concentrations for all molar Ca/Si ratios = 2.7  0 in the C-S-H system, portlandite (CH) for Ca/Si ratios
0 in the C-S-H system, portlandite (CH) for Ca/Si ratios  1.65, congruent dissolution at Ca/Si ratios = 0.85, and amorphous silica (SiO
1.65, congruent dissolution at Ca/Si ratios = 0.85, and amorphous silica (SiO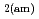 ) for Ca/Si ratios
) for Ca/Si ratios  0.55 as identified in the current study by IR spectroscopy.
0.55 as identified in the current study by IR spectroscopy.
 -ZrO
-ZrO pseudo-binary phase diagram
pseudo-binary phase diagramAlbiol, T.*; Serizawa, Hiroyuki; Arai, Yasuo
Journal of Nuclear Science and Technology, 39(Suppl.3), p.834 - 837, 2002/11
no abstracts in English
Kobayashi, Kaoru*; Kaminaga, Masanori; Haga, Katsuhiro; Kinoshita, Hidetaka; Aso, Tomokazu; Teshigawara, Makoto; Hino, Ryutaro
JAERI-Tech 2002-005, 118 Pages, 2002/02
In order to examine the radiation safety of a spallation mercury target system, it is necessary to clarify the chemical forms of spallation products generated by spallation reaction with proton beam. As for the chemical forms of spallation products in mercury that involves large amounts of spallation products, these forms were estimated by using the binary phase diagrams and the thermochemical equilibrium calculation based on the amounts of spallation product. Calculation results showed that the mercury would dissolve Al, As, B, Be, Bi, C, Co, Cr, Fe, Ga, Ge, Ir, Mo, Nb, Os, Re, Ru, Sb, Si, Ta, Tc, V and W in the element state, and Ag, Au, Ba, Br, Ca, Cd, Ce, Cl, Cs, Cu, Dy, Er, Eu, F, Gd, Hf, Ho, I, In, K, La, Li, Lu, Mg, Mn, Na, Nd, Ni, O, Pb, Pd, Pr, Pt, Rb, Rh, S, Sc, Se, Sm, Sn, Sr, Tb, Te, Ti, Tl, Tm, Y, Yb, Zn and Zr in the form of inorganic mercury compounds.
Nitani, Noriko; Kuramoto, Kenichi; Yamashita, Toshiyuki; Omichi, Toshihiko*
Progress in Nuclear Energy, 38(3-4), p.435 - 438, 2001/02
Times Cited Count:1 Percentile:11.99(Nuclear Science & Technology)no abstracts in English
Yamanaka, Shinsuke*; Abe, Kazuyuki
JNC TY9400 2000-004, 78 Pages, 2000/03
no abstracts in English
Takano, Masahide; Minato, Kazuo; Fukuda, Kosaku; Sato, Seichi*; Ohashi, Hiroshi*
JAERI-Research 97-021, 19 Pages, 1997/03
no abstracts in English
Senda, Ikuo*; Shoji, Teruaki; Nishio, Satoshi; Tsunematsu, Toshihide; *; Fujieda, Hirobumi*
JAERI-Data/Code 95-010, 32 Pages, 1995/08
no abstracts in English
Yoshizawa, Nobuaki*; *; Takada, Hiroshi
Journal of Nuclear Science and Technology, 32(7), p.601 - 607, 1995/07
Times Cited Count:27 Percentile:90.45(Nuclear Science & Technology)no abstracts in English
Matsuda, Toshiaki; *; *; *; *
JAERI-M 90-201, 24 Pages, 1990/11
no abstracts in English
Miwa, Shuhei; Yamashita, Shinichiro; Osaka, Masahiko
no journal, ,
The effects of temperature and atmosphere on cesium (Cs) chemical form just after release from fuel were estimated using chemical equilibrium calculation with consideration of the release kinetics of Cs, iodine (I), boron (B) and so on depending on temperature and atmosphere under a severe accident. The calculation results show that Cs vapor species changed according to temperature and atmosphere, and CsBO vapor species was dominant with the existence of B regardless of temperature and atmosphere.
vapor species was dominant with the existence of B regardless of temperature and atmosphere.
 and SiC in LWR conditions
and SiC in LWR conditionsShirasu, Noriko; Kurata, Masaki
no journal, ,
After Fukushima Daiichi nuclear accident in 2011, enhancing the accident tolerance of light water reactor fuels became a very important issue and currently the development of accident-tolerant fuel (ATF) are in progress. SiC is an attractive candidate of ATF cladding material because of its high chemical stability, high radiation resistance and low neutron absorption. SiC reacts much less than Zircaloy with steam, the generation of heat and hydrogen gas would be extremely suppressed. Thermodynamic evaluation on chemical interactions between UO and SiC were performed on various possible conditions of oxygen potential and temperature in severe accident. SiC is converted to SiO
and SiC were performed on various possible conditions of oxygen potential and temperature in severe accident. SiC is converted to SiO by reaction with O
by reaction with O . SiC is also converted to SiO
. SiC is also converted to SiO by reaction with H
by reaction with H O. However, the fraction of the sub-reaction for forming SiO increases than in the case of reaction with O
O. However, the fraction of the sub-reaction for forming SiO increases than in the case of reaction with O when comparing the results at the same temperatures and the same oxygen potentials.
when comparing the results at the same temperatures and the same oxygen potentials.
Kobayashi, Yutaro*; Haga, Kazuko*; Kaneda, Yoshihisa*; Sato, Tsutomu*; Kakuda, Ayaka; Osugi, Takeshi; Sone, Tomoyuki; Kuroki, Ryoichiro
no journal, ,
no abstracts in English